在《感染性疾病与免疫(英文)》2023年第1期中,福军亮教授团队发表了题为“Effect of Methylprednisolone on Mortality and Clinical Courses in Patients with Severe COVID-19: A Propensity Score Matching Analysis”的论著。内容简介、中英文摘要及作者简介如下:
01
内容简介
本文通过倾向性匹配评分方法,分析了甲基强的松龙治疗对COVID-19重症患者死亡率、核酸转阴时间以及住院时间的影响。研究提示甲基强的松龙治疗未增加重症新冠患者死亡的风险,并且不会延长其核酸转阴时间和总住院日。在所有接受甲基强的松龙治疗的患者中,低剂量、短程治疗组核酸转阴时间和总住院日明显较短。Authors: Xiaoyan Li, Xin Yuan, Zhe Xu, Lei Shi, Lei Huang, Xuechun Lu, Junliang Fu
How to cite this article: Li X, Yuan X, Xu Z, et al. Effect ofmethylprednisolone on mortality and clinical courses in patientswith severeCOVID-19: a propensity score matching analysis. Infect Dis Immun 2023;3(1):20–28. doi: 10.1097/ID9.0000000000000076Funding: This work was supported by grants from National Key R&D Program of China (2020YFC0860900) and Emergency Key Program of Guangzhou Laboratory (EKPG21-30-4).02
中文摘要
背景: 甲基强的松龙治疗对重症COVID-19患者的死亡率、核酸转阴时间和总住院日的影响尚不清楚。本回顾性研究分析了既往的重症COVID-19患者的预后和病程相关数据,为甲基强的松龙治疗重症COVID-19提供更多证据。
方法:回顾性纳入2020年2月至2020年3月武汉市火神山医院和武汉光谷医院收治的4827例确诊COVID-19的患者,其中符合研究标准的重症患者为563例。患者的流行病学及人口学资料、基础疾病、化验结果、主要治疗手段、最终预后和重要的时间节点(如发病、入院、核酸转阴及出院或死亡时间)等信息均从电子病历系统提取。研究主要终点为院内死亡,次要终点为2个临床病程:患者的核酸转阴时间和总住院日。采用单因素和多因素logistic回归及线性回归分析评估甲基强的松龙在不同结局中的作用。采用倾向评分匹配以控制混杂因素。
结果: 本研究纳入的563例患者中接受甲基强的松龙治疗者138例,未接受甲基强的松龙治疗者425例。甲基强的松龙组与非甲基强的松龙组主要结局比较显示,两组间住院死亡率差异有统计学意义(23.91% vs. 1.65%,P<0.001)。在倾向性评分匹配后的分析显示这种死亡率差异仍然存在(13.98% vs. 5.38%,P=0.048)。然而,在匹配后进行的单因素logistic分析显示,甲基强的松龙治疗并非重症患者住院死亡的危险因素(OR 5.242, 95%CI [0.802 - 34.246]; P=0.084)。进一步的多因素logistic回归分析发现,合并基础疾病(OR 3.327, 95%CI [1.702 - 6.501]; P<0.001),低淋巴细胞计数(OR 0.076, 95%CI [0.012 - 0.461]; P=0.005),高乳酸脱氢酶(Lactate dehydrogenase,LDH)水平(OR 1.008, 95%CI [1.003 - 1.013]; P=0.002),以及接受抗凝治疗(OR 11.187, 95%CI [2.459 - 50.900]; P=0.002)与患者的院内死亡结局相关。对次要终点即入院到核酸转阴时间和住院日的分析结果显示,甲基强的松龙治疗也不是核酸转阴时间 (beta 0.081, 95%CI[−1.012 - 3.657]; P=0.265)和总住院日(beta 0.114, 95%CI [−0.723 - 6.408]; P=0.117) 的相关风险因素(图1)。而D -二聚体(beta 0.144, 95%CI [0.012 - 0.817]; P=0.044), LDH (beta 0.260, 95%CI [0.010 - 0.034]; P<0.001),抗病毒治疗(beta 0.220, 95%CI [1.373 - 6.263]; P=0.002)与核酸转阴的延迟有关(图1A)。低淋巴细胞计数(beta −0.206, 95%CI [−6.248 - −1.197]; P=0.004),高LDH (beta 0.231, 95%CI [0.012 - 0.048]; P=0.001),抗病毒治疗(beta 0.143, 95%CI [0.058 - 7.497]; P=0.047),抗感染治疗(beta 0.152, 95%CI [0.133 - 8.154]; P=0.043)与较长的总住院日有关(图1B)。

图1 患者的入院至核酸转阴时间的相关因素(A)及与总住院日时间相关因素(B)
对甲基强的松龙治疗组的分层分析显示,低日剂量组(≤60 mg/d)和低总剂量组(≤200 mg)比相应的高剂量组,其入院至核酸转阴时间 (Z=−2.362, P=0.018; Z=−2.010, P=0.044)和总住院日(Z=−2.735, P=0.006; Z =−3.858, P<0.001)较短(图2)。
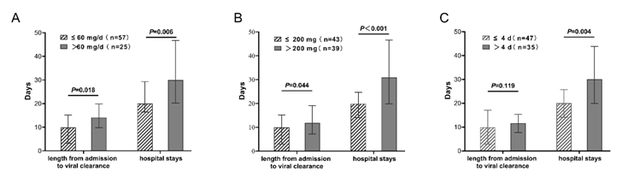
图2 接受甲基强的松龙治疗患者的不同日剂量组(A)、不同总剂量组(B)、不同疗程组(C)的核酸转阴时间及总住院日比较
结论:甲基强的松龙治疗未导致重症COVID-19患者的死亡率增加,也未延长患者从入院到病毒清除时间和总住院日。在治疗组中,低剂量、短期甲基强的松龙治疗可能更有利于缩短病程。
03
Abstract
Background: Whether methylprednisolone therapy can reduce the mortality rate of patients with severe coronavirus disease 2019 (COVID-19) remains controversial, and its effects on the length of hospital stay and virus shedding time are also unknown. This retrospective study investigates the previous issues to provide more evidence for methylprednisolone treatment in severe COVID-19.
Methods: This retrospective study included 563 of 4827 patients with confirmed COVID-19 admitted toWuhan Huoshenshan Hospital or Wuhan Guanggu Hospital between February 3, 2020 andMarch 30, 2020 who met the screening criteria. The participants’ epidemiological and demographic data, comorbidities, laboratory test results, treatments, outcomes, and vital clinical time points were extracted from electronic medical records. The primary outcome was in-hospital death, and the secondary outcomes were 2 clinical courses: length from admission to viral clearance and discharge. Univariate and multivariate logistic or linear regression analyses were used to assess the role of methylprednisolone in different outcomes. Propensity score matching was performed to control for confounding factors.Results: Of the 563 patients who met the screening criteria and were included in the subsequent analysis, 138 were included in the methylprednisolone group and 425 in the nonmethylprednisolone group. The in-hospital death rate between the methylprednisolone and nonmethylprednisolone groups showed a significant difference (23.91% vs. 1.65%, P<0.001), whichwas maintained after propensity score matching (13.98% vs. 5.38%, P=0.048). However, univariate logistic analysis in the matched groups showed that methylprednisolone treatment (odds ratio [OR], 5.242; 95% confidence interval [CI], 0.802 to 34.246; P=0.084) was not a risk factor for in-hospital death in severe patients. Further multivariate logistic regression analysis found comorbidities (OR, 3.327; 95%CI, 1.702 to 6.501; P<0.001), lower lymphocyte count (OR, 0.076; 95%CI, 0.012 to 0.461; P=0.005), higher lactate dehydrogenase (LDH) levels (OR, 1.008; 95% CI, 1.003 to 1.013; P = 0.002), and anticoagulation therapy (OR, 11.187; 95%CI, 2.459 to 50.900; P=0.002) were associated with in-hospitalmortality. Multivariate linear regression analysis in thematched groups showed thatmethylprednisolone treatmentwas not a risk factor for a prolonged duration from admission to viral clearance (b Value 0.081; 95%CI, −1.012 to 3.657; P=0.265) or discharge (b Value 0.114; 95%CI, −0.723 to 6.408; P=0.117). D-dimer (b Value, 0.144; 95%CI, 0.012 to 0.817; P=0.044), LDH (b Value 0.260; 95%CI, 0.010 to 0.034; P<0.001), and antiviral therapy (b Value 0.220; 95%CI, 1.373 to 6.263; P=0.002) were associated with a longer length from admission to viral clearance. The lymphocyte count (b Value −0.206; 95%CI, −6.248 to −1.197; P=0.004), LDH (b Value 0.231;95%CI, 0.012 to 0.048; P=0.001), antiviral therapy (b Value 0.143; 95% CI, 0.058 to 7.497; P = 0.047), and antibacterial therapy (b Value 0.152; 95%CI, 0.133 to 8.154; P=0.043) were associated with a longer hospitalization duration from admission to discharge. Further stratified analysis revealed that the low daily dose group (≤60 mg/d) and the low total dose group (≤200 mg) had shorter duration from admission to viral clearance (Z=−2.362, P=0.018; Z=−2.010, P=0.044) and a shorter hospital stay (Z=−2.735, P=0.006; Z=−3.858, P<0.001).
Conclusions: In patients with severe COVID-19,methylprednisolone is safe and does not prolong the duration from admission to viral clearance or discharge. Low-dose, short-term methylprednisolone treatment may be more beneficial in shortening the disease course.Keywords: COVID-19; Clinical courses; In-hospital death; Methylprednisolone; SARS-CoV-204
作者简介

通讯作者 福军亮 副主任医师 医学博士
解放军总医院第五医学中心感染病医学部感染五病区主任
长期从事慢性肝病以及新突发传染病的临床诊治和转化医学研究工作,承担国家自然科学基金面上项目、国家科技部973子课题、国家卫生部十一五、十二五、十三五重大专项子课题等多项课题研究,以第一或通讯作者在Journal of Hepatology,Gastroenterology,Hepatology,Journal of Immunology, AIDS等相关专业期刊发表多篇论文。

-END-
关注我们

主管单位:中国科协
主办单位:中华医学会
编辑:Infectious Diseases & Immunity编辑委员会
总编辑:王福生
编辑部主任:赵巍
地址:北京市西城区东河沿街69号正弘大厦,100052
网址:http://www.idi-cma.org
邮箱:idi@cmaph.org
电话:010-5132 2080, 010-5132 2086