内容简介
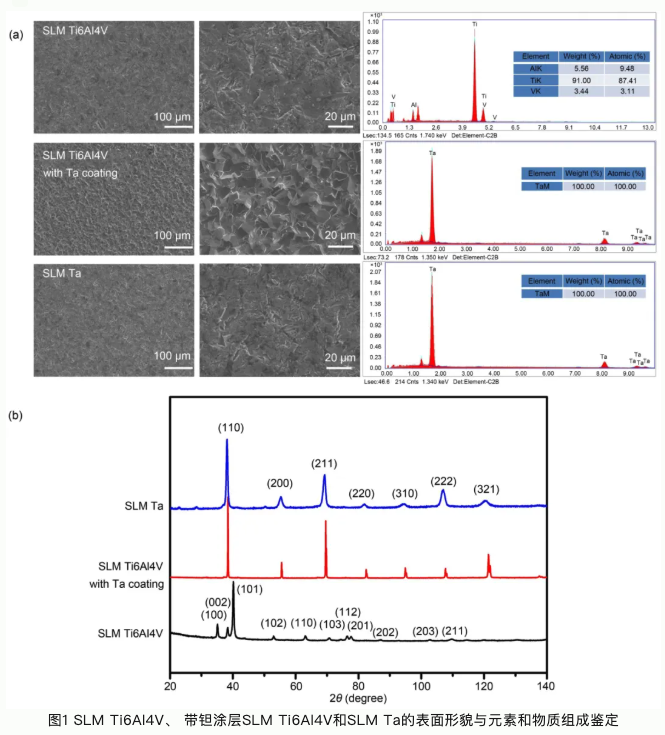
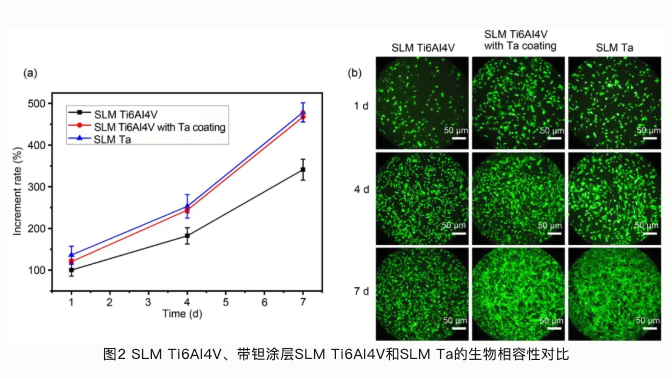
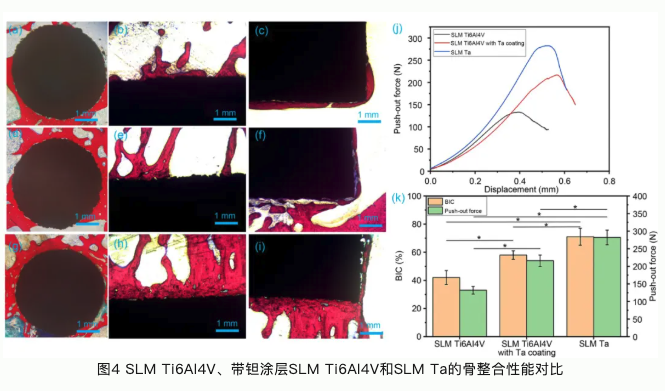
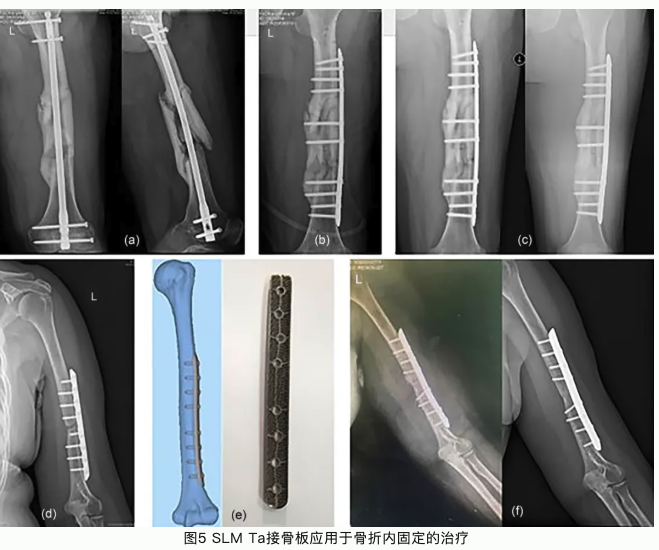
本研究论文聚焦于通过选区激光熔融工艺开发一种生物型钽金属接骨板应用于骨折内固定。选区激光熔融工艺(SLM)是常用的一种应用于金属材料加工的增材制造技术。与传统机械加工技术相比,增材制造技术可以根据患者骨植入部位的解剖结构设计与打印植入体。这种植入体可以完全适配于植入部位骨组织结构和形态。钽金属作为一种亲生物金属,与骨组织具有极佳的亲和力。多孔钽可以引导骨长入,形成骨组织和接骨板一体化结构,具有长期稳定性,不需要二次手术取出;多孔钽可以降低接骨板弹性模量,从而减少应力遮挡效应,加速骨折部位组织修复再生。本论文系统研究了选区激光熔融钽金属、选区激光熔融Ti6Al4V合金以及表面具有化学气相沉积钽涂层的Ti6Al4V合金的理化性能。选区激光熔融钽金属具有良好的综合力学性能,更佳的生物相容性和促成骨相关的生物活性;同时我们还发现钽金属的免疫源性也显著低于Ti6Al4V,有助于减少植入体周围组织炎性,促进骨组织修复再生。临床试验也验证了选区激光熔融钽金属接骨板用于骨折内固定的优势与可行性。

参考文献
1. Han Q, Wang CY, Chen H et al (2019) Porous tantalum and titanium in orthopedics: a review. ACS Biomater Sci Eng 5(11): 5798–5824. https://doi.org/10.1021/acsbiomaterials.9b00493
2. Geetha M, Singh AK, Asokamani R et al (2009) Ti based biomaterials, the ultimate choice for orthopaedic implants-a review. Prog Mater Sci 54(3):397–425. https://doi.org/10.1016/j.pmatsci.2008.06.004
3. Carraro F, Bagno A (2023) Tantalum as trabecular metal for endosseous implantable applications. Biomimetics 8(1):49. https://doi.org/10.3390/biomimetics8010049
4. Li JL, Qin L, Yang K et al (2020) Materials evolution of bone plates for internal fixation of bone fractures: a review. J Mater Sci Technol 36:190–208. https://doi.org/10.1016/j.jmst.2019.07.024
5. Gao HR, Yang JZ, Jin X et al (2021) Porous tantalum scaffolds: fabrication, structure, properties, and orthopedic applications. Mater Des 210:110095. https://doi.org/10.1016/j.matdes.2021.110095
6. Chen QZ, Thouas GA (2015) Metallic implant biomaterials. Mat Sci Eng R 87:1–57. https://doi.org/10.1016/j.mser.2014.10.001
7. Murphy WL, McDevitt TC, Engler AJ (2014) Materials as stem cell regulators. Nat Mater 13(6):547–557. https://doi.org/10.1038/nmat3937
8. Kelly CN, Miller AT, Hollister SJ et al (2018) Design and structure-function characterization of 3D printed synthetic porous biomaterials for tissue engineering. Adv Healthc Mater 7(7):e1701095. https://doi.org/10.1002/adhm.201701095
9. Zhang XY, Fang G, Xing LL et al (2018) Effect of porosity variation strategy on the performance of functionally graded Ti-6Al-4V scaffolds for bone tissue engineering. Mater Des 157:523–538. https://doi.org/10.1016/j.matdes.2018.07.064
10. Chen ZY, Yan XC, Yin S et al (2020) Influence of the pore size and porosity of selective laser melted Ti6Al4V ELI porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Mater Sci Eng C Mater Biol Appl 106:110289. https://doi.org/10.1016/j.msec.2019.110289
11. Ryan G, Pandit A, Apatsidis DP (2006) Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 27(13):2651–2670. https://doi.org/10.1016/j.biomaterials.2005.12.002
12. Davoodi E, Montazerian H, Mirhakimi AS et al (2022) Additively manufactured metallic biomaterials. Bioact Mater 15:214–249. https://doi.org/10.1016/j.bioactmat.2021.12.027
13. Svetlizky D, Das M, Zheng BL et al (2021) Directed energy deposition (DED) additive manufacturing: physical characteristics, defects, challenges and applications. Mater Today 49:271–295. https://doi.org/10.1016/j.mattod.2021.03.020
14. Jia ZJ, Xu XX, Zhu DH et al (2023) Design, printing, and engineering of regenerative biomaterials for personalized bone healthcare. Prog Mater Sci 134:101072. https://doi.org/10.1016/j.pmatsci.2023.101072
15. Javaid M, Haleem A (2018) Current status and challenges of additive manufacturing in orthopaedics: an overview. J Clin Orthop Trauma 10(2):380–386. https://doi.org/10.1016/j.jcot.2018.05.008
16. Zhang XZ, Leary M, Tang HP et al (2018) Selective electron beam manufactured Ti-6Al-4V lattice structures for orthopedic implant applications: current status and outstanding challenges. Curr Opin Solid State Mater Sci 22(3):75–99. https://doi.org/10.1016/j.cossms.2018.05.002
17. Li YF, Liu HW, Wang C et al (2023) 3D printing titanium grid scaffold facilitates osteogenesis in mandibular segmental defects. NPJ Regen Med 8(1):38. https://doi.org/10.1038/s41536-023-00308-0
18. Liu BY, Ma ZJ, Li JL et al (2021) Experimental study of a 3D printed permanent implantable porous Ta-coated bone plate for fracture fixation. Bioact Mater 10:269–280. https://doi.org/10.1016/j.bioactmat.2021.09.009
19. Kunčická L, Kocich R, Lowe TC (2017) Advances in metals and alloys for joint replacement. Prog Mater Sci 88:232–280. https://doi.org/10.1016/j.pmatsci.2017.04.002
20. Bandyopadhyay A, Mitra I, Shivaram A et al (2019) Direct comparison of additively manufactured porous titanium and tantalum implants towards in vivo osseointegration. Addit Manuf 28: 259–266. https://doi.org/10.1016/j.addma.2019.04.025
21. Balla VK, Bodhak S, Bose S et al (2010) Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater 6(8):3349–3359. https://doi.org/10.1016/j.actbio.2010.01.046
22. Avery D, Morandini L, Celt N et al (2023) Immune cell response to orthopedic and craniofacial biomaterials depends on biomaterial composition. Acta Biomater 161:285–297. https://doi.org/10.1016/j.actbio.2023.03.007
23. Gibon E, Amanatullah DF, Loi F et al (2017) The biological response to orthopaedic implants for joint replacement: Part I: metals. J Biomed Mater Res B Appl Biomater 105(7):2162–2173. https://doi.org/10.1002/jbm.b.33734
24. Seyedizade SS, Afshari K, Bayat S et al (2020) Current status of M1 and M2 macrophages pathway as drug targets for inflammatory bowel disease. Arch Immunol Ther Exp 68(2):10. https://doi.org/10.1007/s00005-020-00576-4
25. D’alessio S, Correale C, Tacconi C et al (2014) VEGF-C–dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Investig 124(9):3863–3878. https://doi.org/10.1172/JCI72189
26. Zhu W, Yu JB, Nie Y et al (2014) Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases. Immunol Invest 43(7):638–652. https://doi.org/10.3109/08820139.2014.909456
27. Abaricia JO, Shah AH, Chaubal M et al (2020) Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterial 243:119920. https://doi.org/10.1016/j.biomaterials.2020.119920
28. Donlin LT, Jayatilleke A, Giannopoulou EG et al (2014) Modulation of TNF-induced macrophage polarization by synovial fibroblasts. J Immunol 193(5):2373–2383. https://doi.org/10.4049/jimmunol.1400486
29. Yuan X, Liu W, Li Y et al (2022) CCL3 aggravates intestinal damage in NEC by promoting macrophage chemotaxis and M1 macrophage polarization. Pediatr Res 94(1):119–128. https://doi.org/10.1038/s41390-022-02409-w
30. Ge S, Yang W, Chen HQ et al (2021) MyD88 in macrophages enhances liver fibrosis by activation of NLRP3 inflammasome in HSCs. Int J Mol Sci 22(22):12413. https://doi.org/10.3390/ijms222212413
31. Mitra I, Bose S, Dernell WS et al (2021) 3D printing in alloy design to improve biocompatibility in metallic implants. Mater Today 45:20–34. https://doi.org/10.1016/j.mattod.2020.11.021
